FDA clears first-ever AI for sepsis detection
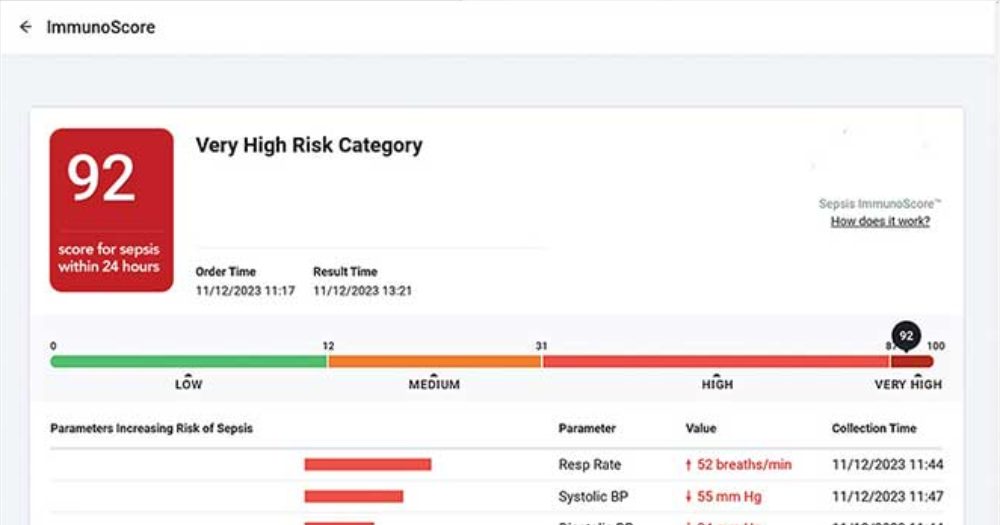
In a groundbreaking announcement, Chicago-based Prenosis — a pioneer in the fusion of AI and precision medicine for acute care scenarios — has recently revealed the Sepsis ImmunoScore's receipt of marketing authorization from the U.S. Food and Drug Administration (FDA) through the De Novo pathway.
This pivotal development heralds the first FDA endorsement for an AI-driven diagnostic instrument aimed at the early detection and prognosis of sepsis, a condition notorious for its diagnostic complexity and substantial impact on healthcare.
How does it work?
The Sepsis ImmunoScore, conceived as an AI-powered software as a medical device (SaMD), fundamentally transforms the approach to assessing sepsis risk. It intricately analyzes a blend of biomarkers and clinical data, harnessing 22 diverse parameters to provide a comprehensive snapshot of a patient's biological status.
Rather than functioning as an alert mechanism, it calculates a risk score distributed across four discrete categories, correlating to the likelihood of clinical deterioration.
This dual capability to diagnose and predict sepsis progression is unprecedented in legally marketed sepsis care tools.
Why does it matter?
Sepsis stands as a colossal challenge, inflicting billions in expenses on the U.S. healthcare system and causing more fatalities annually than all forms of cancer combined.
Prior to the Sepsis ImmunoScore, the healthcare sector lacked an FDA-approved AI tool for sepsis diagnosis, making this authorization not only a technological leap but a beacon of hope for enhancing patient care.
By embedding directly into hospital Electronic Medical Records and providing an intuitive interface for clinicians, the Sepsis ImmunoScore is poised to facilitate rapid, informed treatment decisions, improve patient outcomes, and contribute to more efficient hospital operations.
On the record
"FDA authorization of a sepsis diagnostic tool with significant predictive power is a landmark event for people that could ever be at risk of sepsis at some point in their lives," said Bobby Reddy, Jr, Ph.D., Prenosis Co-Founder and CEO. "Until now, there was no other FDA authorized AI diagnostic for sepsis, which is why the Sepsis ImmunoScore had to be granted marketing authorization through the De Novo pathway. FDA authorization offers yet another important piece of evidence of the potential of the Sepsis ImmunoScore to improve care."
"The Sepsis ImmunoScore FDA authorization marks an important step in helping hospital systems provide better care," added Reddy. "Similar to how sequencing technology enabled the precision medicine revolution in cancer, our powerful Immunix platform has the potential to unlock valuable insights to enable the creation of precision therapeutics guided by AI diagnostics. To date, this type of approach has been used predominantly in healthcare outside of emergency departments and hospitals. Prenosis seeks to change this by catalyzing a personalized medicine revolution in acute care."
The context
The development of the Sepsis ImmunoScore was enabled by Prenosis' Immunix platform, designed to spearhead precision medicine initiatives in acute care. Leveraging a decade-long partnership with ten U.S. hospitals, Prenosis has assembled a comprehensive biobank and dataset, underpinning the largest biological-clinical dataset globally for patients under suspicion of serious infections.
This rich dataset fuels the development of AI algorithms capable of detecting rapid immune response patterns, aiming to personalize therapeutic interventions in real time.
💡Did you know?
You can take your DHArab experience to the next level with our Premium Membership.👉 Click here to learn more
🛠️Featured tool
 Easy-Peasy
Easy-Peasy
An all-in-one AI tool offering the ability to build no-code AI Bots, create articles & social media posts, convert text into natural speech in 40+ languages, create and edit images, generate videos, and more.
👉 Click here to learn more


