EpiWatch Continuous Seizure Monitor gets the green light from FDA
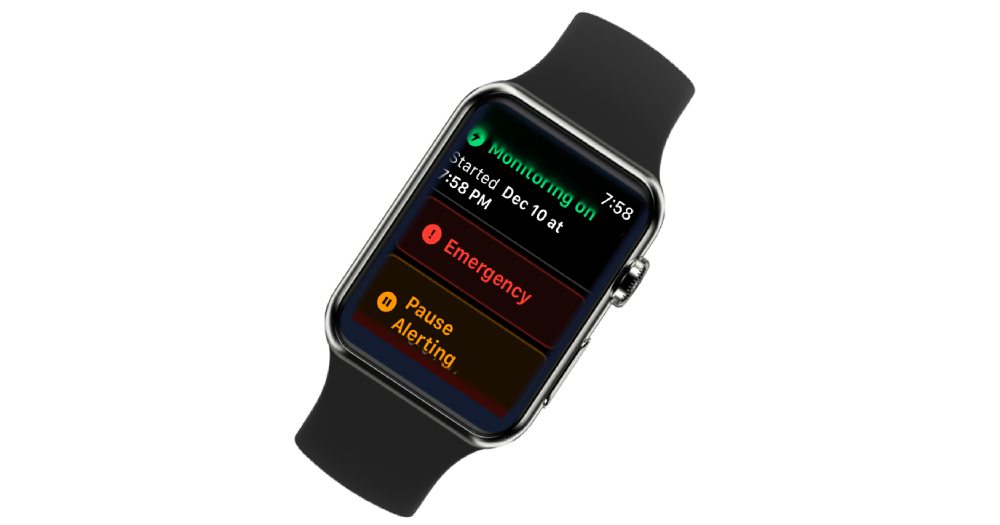
EpiWatch snagged FDA 510(k) clearance for its game-changing seizure detection tech — one that lives right on your wrist. The EpiWatch Continuous Seizure Monitor (CSM) turns the everyday Apple Watch into a life-saving tool, spotting and alerting for tonic-clonic seizures in real time.
As Teresa Prego, EpiWatch CEO, puts it: "This marks a significant step forward in fulfilling our mission to empower all people living with epilepsy... while providing peace of mind for their caregivers and loved ones."
This isn't just another app. It's the first and only FDA-cleared seizure detection app on Apple Watch, built from years of research and clinical trials.
How does it work?
At its heart, EpiWatch is powered by a smart algorithm that tracks physiological signals — think heart rate, movement, and more — to catch signs of tonic-clonic seizures.
Here's what it does:
- Monitors continuously via the Apple Watch
- Detects seizures using a non-EEG signal-based system
- Sends real-time alerts to caregivers, family, and emergency contacts
The tech behind it is discreet — no bulky, condition-specific wearables needed. Just an Apple Watch. That means people can go about their day without drawing attention to their condition.
Why does it matter?
For the 3.4 million Americans with epilepsy, this isn't just another gadget. It's peace of mind. Nearly 40% of these individuals experience uncontrolled or poorly controlled seizures. That's a lot of lives lived in uncertainty.
Timely seizure detection can:
- Improve emergency response
- Support better seizure tracking and management
- Reduce the risk of injury or death
- Potentially help prevent SUDEP (Sudden Unexpected Death in Epilepsy)
In other words, it's not just about data — it's about dignity, independence, and safety. And for families who've lived in fear of "what if," it's a small device that makes a big difference.
The context
EpiWatch isn't just a one-off startup. It spun out of Johns Hopkins University and was developed by two leading epileptologists, Drs. Greg Krauss and Nathan Crone. The app started as part of Apple's ResearchKit, and it's been years in the making.
The clearance follows a major multi-center clinical trial: "EpiWatch: Evaluation of a Non-EEG Physiologic Signal-Based Seizure Monitoring System," which demonstrated a high detection rate with few false alarms — a sweet spot that's tough to hit in seizure tech.
Now, EpiWatch is rolling out to early users and clinicians in a limited market release, aiming to fine-tune education, support, and real-world use. More to come, obviously.
💡Did you know?
You can take your DHArab experience to the next level with our Premium Membership.👉 Click here to learn more
🛠️Featured tool
 Easy-Peasy
Easy-Peasy
An all-in-one AI tool offering the ability to build no-code AI Bots, create articles & social media posts, convert text into natural speech in 40+ languages, create and edit images, generate videos, and more.
👉 Click here to learn more


